Welcome to the shadowy depths of the periodic table, where we shine a light on plutonium, an element as shrouded in controversy as it is indispensable for some of the most powerful applications known to humankind. This elusive metal, with its complex history, has played a pivotal role in shaping the modern world, from the battlegrounds of World War II to the cutting-edge laboratories of today. Understanding the properties and uses of plutonium is not just a journey through the annals of science; it’s a key that unlocks the door to myriad industrial advancements and challenges.
In this article, we’ll embark on an exploratory odyssey to dissect and comprehend the facets of this unique element. From its inception in the minds of visionary scientists to its current standing on the global stage, we aim to provide a comprehensive analysis that covers not just the science, but also the societal implications of this heavy, silvery metal. So buckle up – we’re about to dive deep into the atomic realms where few elements dare to tread.

Plutonium
History of Plutonium
When we journey back to the early 1940s, nestled within the hallowed halls of the University of California, Berkeley, a groundbreaking discovery was brewing. A team led by the brilliant Glenn Seaborg turned the page of the periodic table to a new chapter with their discovery of plutonium in 1940. Imagine the thrill of unearthing an element that had been hiding in plain sight, a treasure on the atomic scale, veiled until human ingenuity uncovered it.
Now, you might wonder, “Why plutonium?” The name resonates with a certain mythological grandeur, doesn’t it? The team, enamored with the tradition of naming elements after planets (uranium after Uranus, neptunium after Neptune), chose to christen their discovery after Pluto, the Roman god of the underworld. It was a nod to the element’s enigmatic and potent nature, as it lay buried in the shadows of scientific knowledge, much like Pluto’s domain in the darkness beyond Neptune.
But the story of plutonium is not just one of scientific triumph. It casts a long shadow over the history of the 20th century with its role in World War II. Plutonium became a key ingredient in the atomic bomb dropped on Nagasaki, etching its name into history books as a harbinger of both awe-inspiring power and profound devastation. The day the “Fat Man” bomb detonated over Nagasaki, plutonium revealed its dual nature to the world: an element that could both fuel humanity’s greatest achievements and its most harrowing moments.
Beyond its ominous wartime usage, the discovery of plutonium marked a watershed moment in nuclear science. It opened up pandora’s box of possibilities by showcasing the might of the atom, both as a formidable weapon and a potential source of energy. Since then, the element has been living a life shrouded in both respect and fear due to its power and potential hazards.
The element’s discovery was not only a pivotal moment in the annals of science but also an illustration of human curiosity and the relentless pursuit of knowledge. It’s a narrative that intertwines with the fabric of our world’s political, military, and scientific tapestry. And as we continue to delve into the story of plutonium, it’s this complexity and duality that make it a truly unique element with a controversial but indelible history.
Physical and Chemical Properties of Plutonium
Imagine, if you will, a metal that doesn’t play by the rules. It’s not shiny like silver yet holds a lustrous charm; it doesn’t stick to one state of being, shifting forms like a chameleon. This is plutonium, a substance that’s as enigmatic as it is unpredictable. Appearing innocuously silvery-gray, this metal’s beauty is skin-deep, hiding a trove of complexities and dangers just beneath the surface.
Plutonium’s physical appearance belies its true nature. It’s radioactive, which means it’s perpetually disintegrating, spewing out particles that can wreak havoc on living cells. But it’s not just its radioactivity that makes it stand out in the periodic table crowd. Plutonium possesses a startling ability to exist in a variety of oxidation states, ranging from Pu(III) to Pu(VII). These differing states allow it to engage in chemical reactions as varied as the personalities at a masquerade ball, making it a particularly versatile, if not somewhat unpredictable, partner in the laboratory.
Handling and storing this element is akin to keeping a dragon in your backyard. It’s theoretically possible, but fraught with challenges. Plutonium’s high radioactivity and toxicity mean that it requires a fortress-like containment to prevent it from impacting human health and the environment. This containment is not just a simple lock and key but a sophisticated system of glove boxes and remote handling tools to protect those daring enough to work with it.
Breaking Down the Beast: Plutonium’s Complexity
- A Schizophrenic Element: Plutonium can have multiple personalities, scientifically speaking. In its various oxidation states, it can be as different from itself as Jekyll is from Hyde, impacting its reactivity and the types of compounds it forms.
- The Heat is On: Another peculiar trait of plutonium is its ability to generate heat as it decays. This property is harnessed in powering the heart of space exploration devices, where plutonium-238 acts as a long-lasting thermal generator.
- A Heavyweight Champion: As a dense metal, with a density of about 19.86 grams per cubic centimeter, it’s significantly more substantial than your average element. This density is a double-edged sword, offering both benefits in terms of nuclear reactions and challenges in transportation and storage.
At the heart of these extraordinary traits lies the atomic structure of plutonium. With 94 protons packed into its nucleus, it is one of the heaviest naturally occurring elements. However, it’s the electrons that are truly the stars of the show. Their configuration allows plutonium to engage in a rich chemistry that is both a boon and a bane to scientists.
With this tantalizing mix of properties, plutonium sits at the crossroads between incredible potential and profound challenges. As it stands, this element is a giant puzzle wrapped in a radioactive enigma, inviting both awe and caution from those who seek to harness its power.
Uses of Plutonium
When we peek into the atomic playbook, plutonium stands out like a heavyweight champion, flexing its muscles in the ring of elements. With a reputation that precedes it, this element has found its way into some of the most critical and, dare we say, explosive sectors of human industry. So, buckle up as we dive into the multifaceted world of plutonium’s applications, where the stakes are as high as the element’s atomic number.
Plutonium’s most infamous role, without a doubt, is its contribution to national defense. It acts as the backbone of nuclear arsenals, where its isotope, Plutonium-239, plays the leading role in nuclear weapons. This isotope is a fissile superstar, capable of sustaining a nuclear chain reaction that releases an immense amount of energy—the kind that reshaped history in Nagasaki back in 1945.
However, it’s not all doom and gloom in the world of plutonium. This element also shines as a beacon of power in the nuclear energy sector. Nuclear reactors, particularly fast-breeder reactors, utilize plutonium as a fuel. Here’s how it works:
- Plutonium-239 captures a neutron and fissions, releasing energy, more neutrons, and smaller nuclei, known as fission products.
- The released neutrons go on to transform more uranium into plutonium, hence the term “breeder,” as it essentially creates its own fuel.
- This process not only generates power but also helps to use up excess uranium, making it a rather resourceful way of energy production.
Think of plutonium as the Swiss Army knife of the periodic table when it comes to its versatility in space travel. The heat that plutonium-238 gives off is like an eternal flame, a beacon in the cold void of space, powering the thermoelectric generators on board spacecraft. These radioisotope thermoelectric generators (RTGs) have been the unsung heroes in missions that have journeyed beyond the reach of solar energy, whispering life into rovers and probes on Mars, and even the Voyager probes, the intrepid explorers of the final frontier.
Yet, the allure of plutonium is not without its controversies. Its potent capacity for destruction and long-lived waste challenges even the most ardent of its advocates. The disposal of plutonium-laden nuclear waste requires a herculean effort, often involving deep geological repositories and robust safeguards. This complexity has made the dialogue around its use as loaded as the element itself.
Despite these concerns, our hunger for innovation keeps us probing into the heart of the atom. The potential of using plutonium in nuclear fusion is a glimmer of hope for a sustainable energy future. Fusion, the process that powers the sun, remains the holy grail of clean energy—yet it’s as elusive as a shadow in a dark room. Scientists are working tirelessly to unlock this source of power, with plutonium possibly playing a part in this cosmic puzzle.
In conclusion, the narrative of plutonium is far from over. Its dual-edged sword cuts deep into the fabric of modern society, powering our cities, defending our nations, and pushing the boundaries of space exploration. As we stand on the cusp of new discoveries, plutonium’s tale weaves into the future tapestry of human achievement, ever so carefully, one atom at a time.
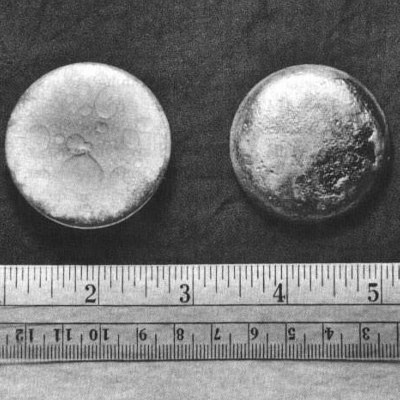
Plutonium
Health and Environmental Impact of Plutonium
Plutonium might sound like a character from a comic book, but in reality, it’s a substance that packs a punch in terms of its health and environmental impact. This radioactive rogue, while useful in many applications, is not one to take lightly when it comes to its potential risks. Let’s dive into the nitty-gritty of how this element can affect us and Mother Earth.
First and foremost, we must consider the invisible threat of radiation. Plutonium emits alpha radiation, a type of particle that, while unable to penetrate the skin, becomes a lethal tango partner if it finds its way inside the human body. Imagine these particles as tiny, uninvited guests at a molecular dance, where they can cause massive disruption and damage. The consequences? A spectrum of health issues, ranging from radiation poisoning to increased risks of cancer, particularly lung, liver, and bone cancer. You definitely don’t want these particles cutting in on your body’s cellular dance.
- Acute exposure can lead to radiation sickness, a nasty affair with symptoms that can include nausea, vomiting, and even hair loss.
- Chronic exposure, on the other hand, can play the long game, leading to cancer and potential genetic damage.
But it’s not just individual health that’s at stake. When it comes to the environment, plutonium is the guest that never leaves. With a half-life stretching anywhere from 24,000 to millions of years, depending on the isotope, addressing the environmental impact is like planning for a very, very, very extended stay. This longevity presents colossal challenges for the containment and isolation of plutonium-laden nuclear waste.
Imagine, if you will, a legacy that outlives human civilization as we know it, with future archaeologists (or aliens, who knows?) stumbling upon our plutonium waste with puzzled antennas or brows. This is why governments and organizations are working tirelessly on methods to safely manage and dispose of plutonium waste. It’s a bit like trying to clean up after a party that’s lasted for several millennia, and nobody’s quite sure where to put the leftover cake that’s become a biohazard.
In the mix of managing plutonium are concerns about the unpredictable tantrums of Mother Nature. Earthquakes, erosion, and unforeseen events can disrupt even the most secure containment systems, potentially releasing this hazardous element back into the environment. The risk here is that it can contaminate soil and water, leading to a chain reaction of ecological upheaval.
So, while the atomic age has brought us impressive technological advances, we’re left with the responsibility of ensuring that our atomic Achilles’ heel doesn’t become a long-term curse. Ongoing research is our beacon of hope, as scientists are looking into new ways to transmute plutonium into less harmful materials or harness its power in safer, more controlled ways. As we ponder the future, we carry the weighty ethical considerations of a world that’s both enriched and endangered by the very same element.
Current Research and Future Possibilities
As we delve deeper into the atomic enigma that is plutonium, we find ourselves standing on the precipice of innovation. The bustling labs and hushed research facilities are a testament to the relentless pursuit of harnessing this element’s full potential. With a twinkle in the eye of science, new methodologies are being developed to foster a future where plutonium isn’t just a word that echoes in the halls of history, but a beacon of progress.
Current research is fervent, with scientists leaning into the winds of challenge, seeking to tame the wild nature of this radioactive beast. One such endeavor is the quest for an alchemy that marries efficiency with sustainability. Researchers are experimenting with advanced reactor designs that could potentially transmute the long-lived isotopes of plutonium into shorter-lived ones. This modern-day wizardry aims to reduce the half-life of nuclear waste, making the storage conundrum less of a herculean task.
- The development of fast breeder reactors promises to use plutonium more efficiently, potentially creating more fuel than they consume.
- Advancements in reprocessing technologies are geared towards minimizing waste and maximizing the use of this precious resource.
- Exploration into molten salt reactors which could offer safer and more stable environments for plutonium-based energy production.
But the plot thickens as we peer beyond the horizon to other industries where plutonium could play a starring role. In the realm of medicine, for instance, the isotope plutonium-238 has already been the lifeblood for cardiac pacemakers. Looking forward, there’s chatter about this power-packed element lighting up the path to powerful diagnostic tools and treatments for diseases.
In the vast void of space, plutonium continues to fuel our extraterrestrial ambitions. The space exploration sector has long relied on plutonium-238 to power spacecraft and rovers on cold, distant worlds where sunlight is scarce. Scientists are now pushing the envelope, brainstorming new ways in which this element can propel us, quite literally, to the stars and beyond.
Challenges and Ethical Considerations
Yet, let’s not gloss over the elephant in the room – the ethical considerations and challenges that accompany the future exploitation of plutonium. The balancing act between leveraging its power and ensuring safety is akin to tightrope walking in a gusty storm. The potential for misuse and the long-term environmental implications of mishaps loom over us, prompting rigorous debate and cautious advancement.
In conclusion, the journey of plutonium is far from over. With scientific curiosity as our compass, we’re charting a course through turbulent waters, aiming for a future where this element’s story is not just one of cautionary tales but also of revolutionary breakthroughs. The narrative of plutonium is still being written, and the next chapters are shimmering with promise, fraught with caution, and brimming with the indomitable spirit of human ingenuity
properties
| Plutonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /pluːˈtoʊniəm/ |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of plutonium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white, tarnishing to dark gray in air | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass number | [244] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Plutonium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | f-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f6 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 24, 8, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 912.5 K (639.4 °C, 1182.9 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3505 K (3228 °C, 5842 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 19.85 g/cm3 (239Pu) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 16.63 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.82 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 333.5 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 35.5 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +2, +3, +4, +5, +6, +7, +8 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 159 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 187±1 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | monoclinic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 46.7 µm/(m⋅K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 6.74 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 1.460 µΩ⋅m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young’s modulus | 96 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 43 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2260 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-07-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after dwarf planet Pluto, itself named after classical god of the underworld Pluto | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, Edwin McMillan (1940–1941) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
