Argon is a colorless, odorless and tasteless gas that is present in the atmosphere. It is a non-toxic and non-reactive element, making it extremely versatile for use in a range of industries and everyday life.
In this comprehensive guide, we explore all you need to know about argon, including its history, properties, uses and safety concerns.
Introduction –
Argon is the third most abundant gas in the Earth’s atmosphere, making up 0.934% of the air we breathe. It is a noble gas, meaning it does not react with most other elements, and it is classified as a non-toxic and non-irritant, making it safe for human exposure. It is often used to create an inert atmosphere for welding and other applications, and is also used in a range of industries and everyday life.
First discovered in the late 19th century, argon has come a long way since then, and its many properties make it incredibly useful in a range of applications. It is relatively inexpensive to produce and is widely available for purchase in pure form. Argon is unique in that it is the only non-reactive element in the atmosphere—it does not react with other elements, and it is also the only noble gas that liquefies at room temperature.
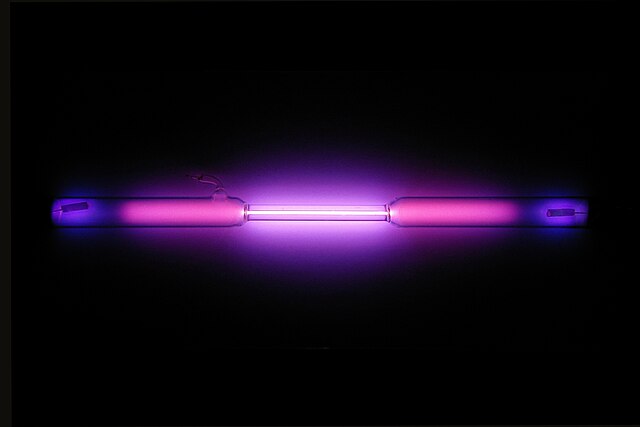
The physical properties of argon are quite interesting. It is the lightest noble gas and has the lowest boiling point of any element. It is also odorless and colorless, and it is insoluble in water. Argon has a very low thermal conductivity, which makes it ideal for insulating applications, as well as cryogenic applications. It is also resistant to most chemicals, making it a useful component in chemical reactions.
Argon has a variety of uses, and is an important component of many industries. It is used in arc welding to produce an inert atmosphere that will help protect against oxidation of the metal being welded. Argon is also used in manufacturing processes, such as creating stainless steel and other alloys, as well as in refrigeration systems. It is also used in scientific research, such as in laboratories to create inert atmospheres for experiments. Additionally, argon is used in everyday life, such as in manufacturing light bulbs, and in producing a variety of food and beverage products.
Although argon is generally considered to be a safe element, there are a few safety concerns that should be taken into account when working with it. It is important to remember that argon is heavier than air and can displace oxygen. Therefore, it is important to ensure that there is adequate ventilation when working with argon-filled containers or in areas where argon is present. It is also important to take proper precautions when working with pressurized containers, and it is important to remember that argon is a flammable gas.
In conclusion, argon is an incredibly useful element with a variety of applications in industry, research and everyday life. It is non-toxic, non-reactive and relatively inexpensive to produce and purchase. Despite its many benefits, there are a few safety concerns that should be taken into account when working with argon. With this comprehensive guide, you now have a better understanding of all things argon and can confidently utilize it in a range of applications.
History of Argon –
Argon is one of the noble gases and was discovered in 1894 through the work of Lord Rayleigh and Sir William Ramsay. Their discovery was made as a result of their attempts to separate the various components of air. During this experiment, they identified a new gaseous element that was odourless, colourless, and non-reactive. They named the new element argon, from Greek meaning “inactive”.
The first recorded use of argon was in 1895 when the Royal Institution in England used the gas to fill incandescent light bulbs. Through further experimentation, Ramsay and Rayleigh discovered that argon and other noble gases, such as helium, neon, and xenon, would not react with other gases or elements. This made argon a valuable tool for scientists to use in experiments and research.
In 1902, argon began to be used in the steel industry as a tool for welding and cutting metals. Argon was used in welding and cutting because it was found to create an inert atmosphere that would protect metals from oxidation. This allowed workers to weld metals that could not be welded in a conventional atmosphere.
In the 1930s, argon was used for the production of glass, as a replacement for nitrogen, to reduce the likelihood of cracking or discoloration. Argon became an essential element for scientists and engineers for a variety of uses. It was also used as a coolant in some nuclear reactors.
In the 1950s, argon began to be used in the production of semiconductors and transistors. This allowed scientists to create smaller and more powerful electronic components than ever before. Argon was also used to create computer chips that were more reliable and powerful than ever before.
In the last few decades, argon has become an essential element for a variety of industries. It is used in cryogenics, laser technology, and spectroscopy. Argon is also used in medical applications, such as MRI and CT scans. Argon continues to be used in a variety of industries and applications, making it an invaluable element for research and industry alike.
Types of Argon –
Argon is a noble gas found in the Earth’s atmosphere to the tune of 0.934% by volume. As a result, it is the third most abundant gas in the atmosphere after nitrogen and oxygen. Argon is also found in the Earth’s lithosphere and hydrosphere, in trace amounts. In its pure state, argon is odorless, colorless and non-toxic.
Natural Sources of Argon: Argon can be found in a variety of sources. It is a naturally occurring gas that is present in the Earth’s atmosphere and is also present in small amounts in the Earth’s lithosphere and hydrosphere. It is also a by-product of the decomposition of organic matter and is present in some combustible fuels.
Uses and Production of Industrial Argon: Argon is used for a variety of industrial purposes, such as welding and cutting metal, filling tires, and for blanketing and purging of inert gases. It is also used for cooling and purging the air in the production of semiconductors and in the manufacture of light bulbs. Argon is produced through fractional distillation of liquid air, which is also used for the production of other noble gases like neon, krypton, and xenon.

Argon is also used in the production of argon-oxygen mixtures for medical purposes. Argon is also used to create non-destructive atmospheres for the storage of food and other sensitive products. Argon is also used as an inert gas shield in arc welding and plasma cutting processes. Argon is used to fill the cavities of certain electronics and to prevent oxidation of certain metal surfaces.
Moreover, argon is used to increase the efficiency of combustion in industrial furnaces and to produce ultra-high purity argon for research and development purposes. Argon’s unique properties make it a valuable gas for many industrial and scientific applications.
In addition to its industrial uses, argon is also used in the production of specialty glasses, such as those used in the production of LCD and LED displays. Argon is also used in some scientific instruments, such as spectrometers and hydrogen sensors.
Argon is also used to fill the cavities of some medical instruments, such as MRI and CT scanners. Argon is also used to make certain types of lasers, such as argon-ion lasers. Argon is also used in some medical research and in the production of certain medical isotopes.
Properties of Argon –
Argon is a colorless, odorless, and tasteless gas, making it one of the most inert elements in the periodic table. It has a low boiling point, and it has a very low solubility in water. It is the third-most abundant element in the Earth’s atmosphere and is found in traces in all other gases. Argon, like other noble gases, has very little interaction with other elements, making it an ideal choice for many applications.
The physical properties of argon are important to consider when using it in industry. For instance, argon has a density of 1.78 g/L, which makes it about 1.4 times heavier than air. Argon is also a non-flammable gas, so it is often used to displace oxygen in enclosed spaces to reduce the risk of fire and explosion. In addition, it is also a good electrical insulator and is used in many applications where electrical insulation is required.
The chemical properties of argon are also worth noting. It is a completely inert element, meaning it has no chemical reactions when exposed to other elements. This makes it ideal for use in industrial settings, as it won’t react with other chemicals. In addition, argon is also non-reactive when exposed to high temperatures, making it a great choice for applications that require high-temperature protection.
Argon is also very stable in its liquid form, which makes it useful for a variety of applications. For instance, liquid argon is often used in cryogenics, as it is able to maintain temperatures at extremely low levels. This is important for many scientific and industrial applications. Argon is also used in welding torches, as its inert nature makes it ideal for welding in oxygen-depleted environments.
Finally, argon is also known for its low level of reactivity with other elements. This means that it is very resistant to corrosion and oxidation, which makes it ideal for use in harsh environments. Argon is also useful in the pharmaceutical industry, as it can be used to store and transport highly reactive or hazardous materials without any risk of contamination.
These are just some of the physical and chemical properties of argon that make it such a valuable element. Its inert nature, low reactivity, and wide range of uses make argon a great choice for many industrial and scientific applications.
Uses of Argon –
Argon has a multitude of uses in industry, research, and everyday life. In industry, argon is used in welding, as an inert gas for safety and protection of workers from toxic and reactive compounds. It is also used in the production of steel and aluminum alloys. In research and science, argon is used to fill vacuum systems, as a protective atmosphere for fragile objects, to study the properties of plasma, and to create an inert atmosphere for hazardous chemical reactions. In everyday life, argon is used in devices such as lightbulbs, as an insulating gas, and in air conditioning systems.
In welding, argon is used as an inert gas for protection and safety. For example, argon is used as a shielding gas to protect welders from toxic and reactive compounds in the air as they weld. In steel and aluminum alloys, argon is used to increase the strength and wear resistance of the alloy. This is because argon is an inert gas that does not react with other compounds.
In research and science, argon plays an important role. It is used to fill vacuum systems due to its low boiling point and non-reactive nature. It is used to create an inert atmosphere for delicate objects, such as a bell jar, which is an airtight glass enclosure used to study the properties of a vacuum. Argon is also used in the study of plasma properties, and to create an inert atmosphere for hazardous chemical reactions.
In everyday life, argon is used in a number of devices. For example, it is used in lightbulbs to create an inert atmosphere. This prevents the filament from reacting with oxygen in the air, which would otherwise reduce its lifespan. Argon is also used as an insulating gas in air conditioning systems and other cooling systems. It can also be used in cryogenic systems, such as liquid nitrogen tanks, to keep the system cool and safe to use.
Argon has a wide range of uses, from welding and metal alloys to research and everyday life. Its inert nature has allowed it to have a variety of applications, and it has been a valuable resource for centuries.
Safety Concerns –
When working with argon, it is important to be aware of its potential risks. Argon is a colorless, odorless and tasteless gas, making it difficult to detect with the naked eye. It is a rather inert gas, making it safe to use in most applications. However, it can be extremely hazardous if mishandled or if proper safety precautions are not followed.
Air with a high concentration of argon has been known to cause headaches, dizziness, and nausea, and can lead to asphyxiation if breathed in at a high enough concentration. Therefore, it is important to ensure that the area where argon is being handled is well ventilated and monitored. In addition, all workers should be well-trained in the proper use of safety equipment such as respirators and protective clothing.
Argon is also flammable and can cause explosions if it comes into contact with a source of ignition. Therefore, it is important to ensure that no sources of ignition are present in areas where argon is being handled. In addition, it is important to avoid any sparks or flames, as well as any high-energy activities such as grinding or welding.
Argon can also be corrosive and can cause damage to metals and other materials if it is improperly handled. Therefore, it is important to ensure that all materials that will be exposed to argon are compatible with it. In addition, it is important to make sure that all equipment used for handling argon is in good condition and well maintained.
Finally, it is important to ensure that all safety procedures are properly followed when dealing with argon. This includes following all relevant laws and regulations, as well as developing and implementing a safety plan. Additionally, it is important to ensure that all workers have received the necessary training and are aware of the potential risks associated with working with argon. Following these safety precautions can help to ensure that working with argon is done safely and without any risks.

Information
| Argon | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | AR-gon | |||||||||||||||||||||||||||||||||||||||||||||
| Appearance | colorless gas exhibiting a lilac/violet glow when placed in an electric field | |||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Ar) | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Argon in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 18 | |||||||||||||||||||||||||||||||||||||||||||||
| Group | group 18 (noble gases) | |||||||||||||||||||||||||||||||||||||||||||||
| Period | period 3 | |||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p6 | |||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 8 | |||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | gas | |||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 83.81 K (−189.34 °C, −308.81 °F) | |||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 87.302 K (−185.848 °C, −302.526 °F) | |||||||||||||||||||||||||||||||||||||||||||||
| Density (at STP) | 1.784 g/L | |||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at b.p.) | 1.3954 g/cm3 | |||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 83.8058 K, 68.89 kPa | |||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 150.687 K, 4.863 MPa | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 1.18 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 6.53 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 20.85 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
|
||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 0 | |||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: no data | |||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 106±10 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 188 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) | |||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 323 m/s (gas, at 27 °C) | |||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 17.72×10−3 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −19.6×10−6 cm3/mol | |||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-37-1 | |||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Lord Rayleigh and William Ramsay (1894) | |||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
Facts
Argon is a chemical element with the symbol Ar
Argon has the atomic number 18
It is in group 18 of the periodic table and is a noble gas
Argon is the third-most abundant gas in Earth’s atmosphere, at 0.934%
Argon is the most abundant noble gas in Earth’s crust, comprising 0.00015% of the crust.
Nearly all of the argon in Earth’s atmosphere is radiogenic argon-40
The name “argon” is derived from the Greek word ἀργόν, neuter singular form of ἀργός meaning ‘lazy’ or ‘inactive’
Argon is extracted industrially by the fractional distillation of liquid air.
Argon is also used in incandescent, fluorescent lighting, and other gas-discharge tubes
Argon makes a distinctive blue-green gas laser.
Argon is colorless, odorless, nonflammable and nontoxic as a solid, liquid or gas
Argon was first isolated from air in 1894 by Lord Rayleigh and Sir William Ramsay at University College London
Earth’s crust and seawater contain 1.2 ppm and 0.45 ppm of argon, respectively
The main isotopes of argon found on Earth are 40Ar (99.6%), 36Ar (0.34%), and 38Ar (0.06%).
The atmospheres of Mars, Mercury and Titan (the largest moon of Saturn) contain argon
About 700,000 tonnes of argon are produced worldwide every year
Argon is the cheapest alternative when nitrogen is not sufficiently inert.
Argon’s density can displace oxygen, which makes it a common component in a fire-suppression system.
Argon is added into incandescent light bulbs to protect the filament from oxidation.
