Cobalt is a naturally occurring metal with a wide variety of uses. It is an essential part of the modern world and has an interesting history, and many of the properties and uses of cobalt have made it a valuable commodity.
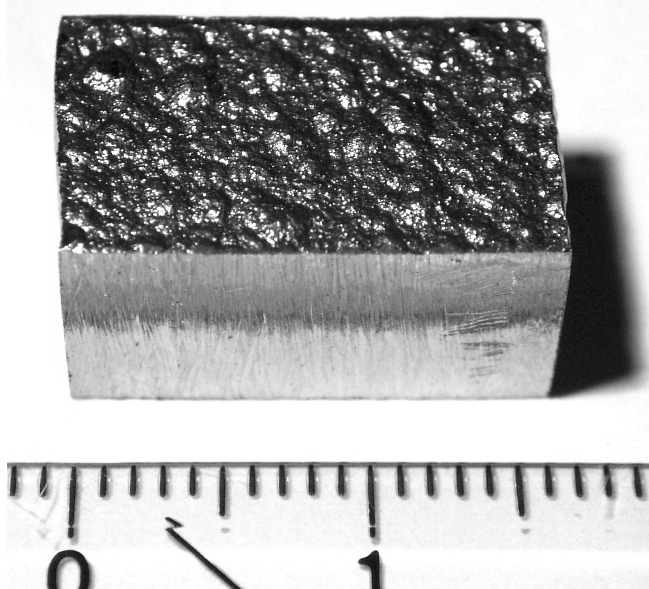
Cobalt is a transition metal found in the d-block of the periodic table and can form a wide variety of compounds. It is silvery grey in color and has a high melting point of 1495°C. Cobalt is generally obtained through mining, but can also be produced through refining and processing.
Cobalt has a wide variety of industrial applications, from being used in jet engines and spacecraft components to medical implants and batteries. It is also an essential trace element for many living organisms. Cobalt is often used to create alloys, such as steel, and can also be found in many different paints and pigments.

Kobalt
The cobalt market is highly dynamic, with its price fluctuating due to a number of factors, including the global supply and demand for cobalt, and the different uses of cobalt around the world. The top cobalt producing countries are mainly found in Africa, including the Democratic Republic of Congo, Zambia and Madagascar. Major cobalt consumers include China, the United States, and Japan.
This guide will provide a comprehensive overview of cobalt, covering its benefits, history, properties, uses, production, safety, and current market. It is designed to be a definitive source of information on cobalt, allowing readers to gain a detailed understanding of this valuable metal and its role in the modern world.
Properties of Cobalt
Cobalt is an important metal with unique properties that make it useful in many different industries. It is a hard, greyish-white metal that has a wide range of mechanical, magnetic, and chemical properties.
On the Mohs scale of hardness, cobalt ranks just above iron at 5.5. This makes it an ideal metal for a variety of applications, from industrial to medical. Cobalt is also highly resistant to corrosion and wear, making it a great choice for machinery components that need to endure harsh conditions.
Cobalt is a ferromagnetic metal, meaning it is highly magnetic and can be used to create strong magnetic fields. This property makes it useful for a wide range of applications, from medical imaging to magnetic recording.
The chemical composition of cobalt consists of cobalt, chromium, and molybdenum. These elements combine to give cobalt its unique properties, including its strength, hardness, corrosion resistance, and magnetic properties.
The physical and magnetic properties of cobalt can vary depending on the chemical composition of the metal. Cobalt alloys are often used to create higher strength and magnetic field strength than pure cobalt.
Cobalt is also highly resistant to wear and corrosion. The metal is extremely resistant to oxidation and corrosion from seawater, acids, and other chemicals. This makes cobalt a great choice for machinery components that are exposed to harsh conditions.
Cobalt’s unique properties make it a useful and versatile metal for a wide variety of applications. Its strength, corrosion resistance, and magnetic properties make it ideal for industrial, medical, and automotive uses.
Uses of Cobalt
Cobalt is a highly versatile element that has many different uses in a variety of industries. It has excellent strength and corrosion resistance properties, making it a key component in many products. In the industrial sector, cobalt is used to enhance the performance of alloys, while in the medical sector it is used for radiation therapy. Cobalt is also used in the automotive sector to produce strong, heat-resistant components.
Industrial Uses:
Cobalt is primarily used in the industrial sector due to its excellent strength and corrosion resistance properties. It is used to enhance the performance of alloys, as it helps increase strength, ductility, and hardenability. Cobalt is also used to produce magnets, as it creates stronger magnetic fields than other elements. Cobalt is also used in the production of batteries, as it is capable of storing large amounts of energy.
Medical Uses:
Cobalt is used in the medical sector for radiation therapy. Cobalt-60, a radioactive isotope of cobalt, is used in the form of radiation therapy. This type of radiation is used to target cancer cells and destroy them without damaging the surrounding healthy cells. Cobalt-60 is also used in radiography, which is a non-invasive imaging technique used to diagnose illnesses.
Automotive Uses:
Cobalt is also used in the automotive sector to produce strong, heat-resistant components. It is used to produce high-performance components such as engine valves and turbochargers. In addition, cobalt is used to produce electrodes and catalytic converters, which help reduce harmful emissions.
Cobalt is a highly versatile element and is used in many industries due to its excellent strength and corrosion resistance properties. In the industrial sector, cobalt is used to enhance the performance of alloys, while in the medical sector it is used for radiation therapy. In the automotive sector, cobalt is used to produce strong, heat-resistant components. All of these industries benefit from the numerous advantages that cobalt has to offer.
Cobalt Production
When it comes to cobalt production, there are three main steps involved in the process: mining, processing, and refining. Each of these stages is essential for obtaining the purest cobalt for industrial applications.
Mining Cobalt:
The first step in cobalt production is mining. The majority of cobalt is mined from copper-cobalt deposits in Africa, with the Democratic Republic of Congo being the largest producer. Other producers include Russia, Canada, and Australia.
In the mining process, ore is extracted from the ground and transferred to a processing plant. The ore is then crushed and the cobalt is separated from other metals found in the ore.
Processing Cobalt:
Once the cobalt has been separated from the ore, it is sent for further processing. At this stage, the cobalt is converted into a cobalt concentrate. The concentrate is then passed through a furnace, where the cobalt is separated from other metals.
The cobalt is then further processed, usually by roasting or leaching, to produce pure cobalt. This process removes impurities and produces a cobalt-rich product that is ready for refining.
Refining Cobalt:
The final step in cobalt production is refining. The cobalt-rich product is put through a refinery, where the cobalt is further purified and then formed into different shapes and sizes. This step ensures that the cobalt is of the highest quality and is ready for industrial applications.
Overall, the three steps of mining, processing, and refining are essential for producing the purest cobalt for industrial use. This process is used to produce cobalt for various applications, such as medical, automotive, and industrial purposes. The quality of the cobalt produced in this process is essential for its end usage.
Cobalt Safety
Cobalt is an essential element in many industrial applications, but its safety must be taken into account. Understanding the health effects of cobalt exposure, as well as the environmental impact of cobalt production and use, is an important part of managing cobalt-related safety concerns.
Health Effects of Cobalt
Breathing in cobalt dust or working with cobalt-containing compounds can cause adverse health effects. Long-term exposure to cobalt dust or fumes can cause difficulty breathing, coughing, and wheezing. It can also cause irritation of the eyes and skin. In extreme cases, it can cause cobalt poisoning or “hard metal lung disease”, which is caused by inhaling cobalt-containing particles.
At higher levels, cobalt can cause neurological effects, including tremors, difficulty walking, and loss of coordination. It can also cause cancer of the lung and other organs. Furthermore, some studies have suggested that cobalt can damage the reproductive system and cause birth defects.
Environmental Impact of Cobalt
Cobalt production, especially mining, can have a significant environmental impact. The mining process can cause air and water pollution, and can lead to the destruction of ecosystems and habitats. Cobalt mining can also cause soil and water contamination, which can be harmful to humans and wildlife.
Cobalt production also creates waste products, which can be harmful if not disposed of properly. For this reason, strict regulations exist to ensure that cobalt producers adhere to environmental standards.
Cobalt Safety Regulations
It is important to note that while cobalt is an essential element, it can also be dangerous if not handled properly. For this reason, strict safety guidelines and regulations have been put in place to ensure that cobalt is handled and used safely.
At the global level, International Labour Organization (ILO) regulations exist to protect workers from the health risks of cobalt exposure. Countries also have their own regulations that must be followed for the safe handling and use of cobalt. These regulations include guidelines for the use of protective equipment, storage, disposal, and exposure limits.
It is important that cobalt producers, users, and handlers adhere to these safety regulations to protect themselves, their colleagues, and the environment from the potential dangers of cobalt exposure and pollution.
Current Market for Cobalt
Cobalt is an essential element and a commodity that is highly sought after in many industries. As such, the cobalt market is an important one that is closely monitored by investors and manufacturers alike. The current market for cobalt is highly volatile, meaning that prices can shift quickly and dramatically depending on global supply and demand.
In recent years, cobalt prices have been skyrocketing due to increased demand and limited global supply. The price of cobalt has increased by more than 120% since 2016, and by nearly 500% since 2014. This surge in prices has been driven by increased demand for cobalt in the production of lithium-ion batteries, as well as revitalized demand from traditional cobalt-consuming markets.
In order to understand the cobalt market, it is important to look at the top cobalt-producing and cobalt-consuming countries. The Democratic Republic of the Congo (DRC) currently dominates the cobalt production market, producing more than 70% of the world’s cobalt. Other top cobalt-producing countries include Canada, Zambia, Russia, and Cuba.
The top cobalt-consuming countries are China, Japan, and the United States. China is the largest cobalt-consuming nation, consuming more than 50% of the global cobalt market share. In the United States, cobalt consumption is mainly driven by the automotive industry, while in Japan it is driven by the electronics industry.
It is important for investors and manufacturers to be aware of the cobalt market’s volatility, and to be prepared for sudden shifts in supply and demand. Due to the limited global supply of cobalt, any changes in supply can lead to dramatic changes in prices. As such, it is important to monitor global developments and trends in order to understand the current state of the cobalt market.
Facts
Cobalt has the symbol Co
Cobalt has the atomic number 27
Cobalt became the first metal to be discovered since the pre-historical period
The word cobalt is derived from the German kobalt, from kobold meaning “goblin”
59Co is the only stable cobalt isotope and the only isotope that exists naturally on Earth
There are twenty-two known radioactive isotopes of cobalt.
Swedish chemist Georg Brandt is credited with discovering cobalt around 1735
Cobalt is a ferromagnetic element.
Trace amounts of cobalt are found in most soil samples, minerals, and rocks.
It makes up about 0.0029% of the planet’s crust.
The smelting process of cobalt can release arsenic vapors.
The main ores of cobalt are cobaltite, erythrite, glaucodot and skutterudite
The Democratic Republic of the Congo currently produces 63% of the world’s cobalt
Cobalt is principally mined as a by-product of nickel and copper mining.
Cobalt compounds have been used for centuries to impart a rich blue color to glass, glazes, and ceramics
Cobalt has been used to color glass since the Bronze Age.
Cobalt has been detected in Egyptian sculpture
Cobalt has been detected in the ruins of Pompeii
Cobalt is primarily used in lithium-ion batteries
Cobalt is essential to the metabolism of all animals.
It is a key constituent of cobalamin, also known as vitamin B12
Cobalt is used in alloys for aircraft engine parts and in alloys with corrosion/wear resistant uses.
Cobalt is also used in the petroleum industry as a catalyst when refining crude oil
Cobalt-60 is a commercially important radioisotope, used as a radioactive tracer
Info
| Cobalt | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈkoʊbɒlt/ | |||||||||||||||||||||||||||||||||||
| Appearance | hard lustrous bluish gray metal | |||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Co) | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Cobalt in the periodic table | ||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 27 | |||||||||||||||||||||||||||||||||||
| Group | group 9 | |||||||||||||||||||||||||||||||||||
| Period | period 4 | |||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d7 4s2 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 15, 2 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||
| Melting point | 1768 K (1495 °C, 2723 °F) | |||||||||||||||||||||||||||||||||||
| Boiling point | 3200 K (2927 °C, 5301 °F) | |||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.90 g/cm3 | |||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 7.75 g/cm3 | |||||||||||||||||||||||||||||||||||
| Heat of fusion | 16.06 kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | 377 kJ/mol | |||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.81 J/(mol·K) | |||||||||||||||||||||||||||||||||||
Vapor pressure
|
||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | −3, −1, 0, +1, +2, +3, +4, +5 (an amphoteric oxide) | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.88 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
|
|||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 125 pm | |||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 126±3 pm High spin: 150±7 pm |
|||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | |||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 4720 m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||
| Thermal expansion | 13.0 µm/(m⋅K) (at 25 °C) | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 100 W/(m⋅K) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 62.4 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | |||||||||||||||||||||||||||||||||||
| Young’s modulus | 209 GPa | |||||||||||||||||||||||||||||||||||
| Shear modulus | 75 GPa | |||||||||||||||||||||||||||||||||||
| Bulk modulus | 180 GPa | |||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.31 | |||||||||||||||||||||||||||||||||||
| Mohs hardness | 5.0 | |||||||||||||||||||||||||||||||||||
| Vickers hardness | 1043 MPa | |||||||||||||||||||||||||||||||||||
| Brinell hardness | 470–3000 MPa | |||||||||||||||||||||||||||||||||||
| CAS Number | 7440-48-4 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Georg Brandt (1735) | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
