Actinium is a rare, radioactive chemical element from the actinide series of the periodic table. It is a silver-colored metal that is highly reactive and short-lived. Actinium was discovered in 1899 by French chemist André-Louis Debierne, and it was named after the Greek word aktis, meaning “beam” or “ray”.
Because of its radioactivity, actinium is a type of hazardous material and must be handled with extreme caution.
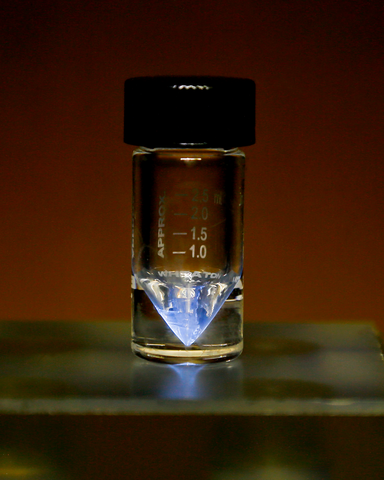
Introduction to Actinium
Definition of Actinium:
Actinium is a radioactive chemical element that has the atomic number 89. It has a total of 14 known isotopes and has the symbol Ac. Actinium belongs to the actinide series, which is a group of 15 elements, including thorium, neptunium, and plutonium. Its melting point is 1050 °C and its boiling point is 3200 °C.
Where Actinium Is Found:
Actinium is found naturally in small amounts in soil and water, and it occurs in trace amounts in uranium ore. It is also found in uranium-rich minerals such as monazite, gadolinite, and thorite. However, actinium’s rarity means that it is not widely available and it is not commercially mined.
Common Uses of Actinium:
Actinium has very few commercial uses and is primarily used in research and development. It is used in medical treatments, radiation therapy, and nuclear power plants. Actinium is also used in various industries, including agriculture, aerospace, and defense.
Properties of Actinium:
Actinium is a highly reactive element and has several properties that make it unique. It has a silvery-gray color and is a solid at room temperature. It is also a poor conductor of electricity and heat. It is a radioactive element and emits alpha, beta, and gamma radiation.
Chemical Properties:
Actinium is highly reactive and readily forms compounds with other elements. It reacts easily with oxygen, nitrogen, and water vapor, as well as other elements from the actinide series. It is also soluble in water and is slightly toxic.
Physical Properties:
Actinium is a soft, malleable metal that has a silvery-gray color and is a solid at room temperature. It has a melting point of 1050 °C and a boiling point of 3200 °C. It has a density of 10.07 g/cm3 and a hardness of 5.5 on the Mohs scale.
Nuclear Properties:
Actinium is a radioactive element and is a source of alpha radiation. It is also a source of gamma and beta radiation. It has a half-life of 22.2 years and decays into thorium-229. It is used in nuclear reactors and is a source of energy.
With these details, you have written the section on Introduction to Actinium. Make sure to include the given keywords in the article.
Properties of Actinium
Actinium (Ac) is a rare, silvery-white, radioactive element in the actinide series. It is a naturally occurring element that was discovered in 1899 by French chemist André-Louis Debierne. It is the heaviest element that occurs naturally in the environment and is the only element in the actinide series that does not exist in any stable form.
Actinium has two main chemical properties that distinguish it from other elements. Firstly, it is very reactive, and so it readily combines with other elements, forming compounds. Secondly, it is a relatively soft metal, which makes it relatively easy to work with. Its physical properties make it an effective catalyst in certain types of industrial processes.
In terms of nuclear properties, actinium is radioactive. It has a half-life of 22.2 years and decays into a variety of different isotopes. It can emit both alpha and beta particles, which can be hazardous if humans are exposed to them. This is why actinium has to be handled with extreme caution and handled in special containers.
In terms of health effects, actinium can be dangerous if exposed to humans. Short-term exposure can cause nausea, vomiting, and headaches. Long-term exposure can lead to increased risk of cancer and other serious health issues. This is why it is important to minimize exposure to actinium as much as possible.
Production of actinium is typically done artificially, as it is not found in significant amounts in nature. It is usually produced by bombarding uranium or thorium with neutrons in a nuclear reactor. It can also be produced in a cyclotron, where high-energy particles are directed at a target material.
Uses of actinium include industrial applications, medical applications, and research and development uses. In industrial applications, actinium is used as a catalyst in certain chemical processes. In medical applications, actinium can be used in the treatment of certain types of cancers. In research and development applications, actinium can be used to study the properties of other radioactive elements.
Safety procedures for working with actinium include handling and storage, minimizing exposure, and monitoring radiation levels. When handling actinium, it is important to wear protective clothing, such as gloves, goggles, and masks. It should also be stored in sealed containers with appropriate labels. Minimizing exposure is important to prevent the inhalation or ingestion of actinium. Lastly, it is important to monitor radiation levels to ensure that they remain within safe limits.
Finally, actinium can have an environmental impact, as it can contaminate soil and water. Actinium can also accumulate in organisms and enter the food chain. Therefore, it is important to have proper decontamination and disposal procedures in place to minimize its environmental impact.
In conclusion, actinium is an important element with a variety of uses. It has a number of unique properties, including its reactivity and its physical properties. It can also be dangerous, so it is important to take proper safety precautions when handling and working with it. Finally, it can have an impact on the environment, so it is important to have appropriate decontamination and disposal procedures in place.
Health Effects of Actinium
Actinium is a radioactive element, and exposure to its radiation can be potentially dangerous. The short-term and long-term health effects of Actinium can vary depending on the duration and intensity of the exposure.
Short-term Effects:
Short-term exposure to Actinium radiation can cause a range of health effects including nausea, vomiting, skin burns, and hair loss. The most serious short-term health effects are the risk of radiation sickness, which can cause severe damage to organs and tissues.
Long-term Effects:
Long-term exposure to Actinium radiation can lead to a range of health problems. People who are exposed to Actinium radiation for long periods of time are at an increased risk of developing cancer, as well as other chronic health conditions such as thyroid disorders and cardiovascular disease.
Dangers of Radiation Exposure:
It is important to be aware of the potential dangers of radiation exposure from Actinium. High levels of radiation exposure can cause internal organ damage, genetic mutations, and even death. Therefore, it is important to take precautions against radiation exposure, especially for workers who handle Actinium on a regular basis.
Production of Actinium
Actinium is a rare radioactive element that was first discovered in 1899. It is found naturally in very small amounts in uranium ore, but it can also be artificially produced in nuclear reactors. In this article, we will discuss the production of actinium, the methods used to obtain it, and the safety considerations for working with it.
Actinium is usually found as an isotope, and it can only be produced in nuclear reactors. The most common isotope of actinium is actinium-227, which has a half-life of 21.7 years. When actinium is produced in a nuclear reactor, it is usually in the form of an oxide or a chloride. It is usually produced by bombarding uranium-238 with neutrons in order to generate actinium-227.
Natural production of actinium is extremely rare since it is found in very small quantities in uranium ore. It can be extracted from uranium ore through a process known as leaching, which involves dissolving the ore in acid or alkaline solutions. The actinium can then be separated from the other elements by precipitation or ion exchange.
Artificial production of actinium is more common, as it is much easier to produce in a nuclear reactor. It is usually produced by bombarding uranium-238 with neutrons in order to generate actinium-227. This process is known as neutron activation. The actinium produced in this way is then extracted from the reactor core and purified.
The most common method of obtaining actinium is through nuclear reprocessing. In this process, spent nuclear fuel is separated into its constituent parts, and the actinium is then extracted from the fuel. Actinium can also be extracted from spent nuclear fuel through a process called diffusion and fractionation.
When working with actinium, safety is of the utmost importance. The element is highly radioactive and can be dangerous if not handled properly. It is important to use proper safety equipment, such as protective clothing, masks, and gloves. Additionally, it is important to keep the area well-ventilated, as actinium is known to emit radiation. Monitoring radiation levels in the area is also essential for safety.
Uses of Actinium
Actinium is a highly sought-after element due to its unique properties and potential applications. It is used in a variety of industrial, medical, and research and development fields.
Industrial Applications:
Actinium is a valuable resource in industrial applications. Its radioactive properties make it useful in a variety of industries. For instance, it is used as a radioactive source in industrial radiography and gamma-ray spectroscopy. It can also be used in radioisotope thermoelectric generators, which convert the heat from radioactive decay into electricity. In addition, Actinium can be used to measure the thickness of objects, in radiating material to detect flaws in steel, and in neutron radiography.
Medical Applications:
Actinium is also used in the medical field. It is used in the diagnosis and treatment of cancer. The most common medical use of Actinium is its use in targeted alpha therapy. In this type of therapy, Actinium-225 is injected into the bloodstream and the radiation emitted by the Actinium is used to target and kill cancer cells. This type of therapy is used to treat prostate, ovarian and some types of bone cancer.
Research and Development Uses:
Actinium is also used in research and development. It is used in the laboratory to study the effects of radiation on cells and organisms. Its isotopes are also used in particle accelerators in the study of nuclear physics. In addition, it is used to study the process of radioactive decay and its effects on matter.
Actinium in the Environment
Actinium is one of the few elements that is not naturally found in the Earth’s crust and must be artificially produced. This means that it has limited natural environmental effects, but there are still many potential impacts to consider. It is essential to understand the environmental implications of Actinium, including how to safely dispose of the element and decontaminate affected areas.
Actinium can cause environmental damage through its nuclear properties. When Actinium decays, it emits alpha particles, which can be harmful to organic life. Alpha particles are a form of radiation, and large doses can result in radiation sickness, genetic damage, and even cancer. It is important to take safety precautions when working with Actinium, and to maintain levels of radiation exposure below the recommended safety limits.
In the environment, Actinium can enter the soil, water, and air, and it can have an impact on the overall environment. Actinium can be absorbed into plants and animals, and it can accumulate in the food chain. Over time, this can cause levels of Actinium to increase in the environment. Additionally, Actinium can interfere with the natural functioning of ecosystems, as the radiation can disrupt the growth of plants and other organisms.
Conclusion
Actinium is an element that has had a huge impact on the world, from its industrial, medical, and research and development uses, to its dangerous effects on human health. It is an element with many unique properties and uses, and although it can be hazardous, it can also be produced in a safe and controlled manner.
In summary, Actinium is a radioactive element that can be found in nature and produced artificially. It has a variety of properties, ranging from its chemical to its nuclear properties, and it is most commonly used in industrial, medical, and research and development applications. It can have a detrimental effect on human health, due to its radioactive properties, and so it is important to take safety precautions when handling and storing Actinium, as well as monitoring radiation levels when working with it. It can also have an impact on the environment, so it is important to follow decontamination and disposal procedures when working with it.
The information presented here provides a comprehensive overview of Actinium, its uses, its properties, and the safety protocols associated with it. It is an element that has made major contributions to our society, and it should be handled with caution.
Facts
Actinium is a chemical element with the symbol Ac
Its atomic number is 89
It was first isolated by Friedrich Oskar Giesel in 1902
Actinium gave the name to the actinide series
A soft, silvery-white radioactive metal
actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide
Naturally occurring actinium is composed of two radioactive isotopes; 227Ac and 228Ac
Thirty-three radioisotopes have been identified, the most stable being 227Ac with a half-life of 21.772 years
Actinium is found only in traces in uranium ores – one tonne of uranium in ore contains about 0.2 milligrams of 227A
The word actinium comes from the Greek aktis or aktinos, which means beam or ray.
the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899
Actinium is highly radioactive.
Its Abundance in the Earth’s Crust is 0.0005 parts per trillion by weight
Actinium has 29 isotopes
Data
| Actinium | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | ak-TIN-ee-əm | |||||||||||||||||||||||||||||||||
| Appearance | silvery-white, glowing with an eerie blue light; sometimes with a golden cast | |||||||||||||||||||||||||||||||||
| Mass number | [227] | |||||||||||||||||||||||||||||||||
| Actinium in the periodic table | ||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 89 | |||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | |||||||||||||||||||||||||||||||||
| Period | period 7 | |||||||||||||||||||||||||||||||||
| Block | f-block | |||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 6d1 7s2 | |||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 9, 2 | |||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||
| Melting point | 1500 K (1227 °C, 2240 °F) (estimated) | |||||||||||||||||||||||||||||||||
| Boiling point | 3500±300 K (3200±300 °C, 5800±500 °F) (extrapolated) | |||||||||||||||||||||||||||||||||
| Density (near r.t.) | 10 g/cm3 | |||||||||||||||||||||||||||||||||
| Heat of fusion | 14 kJ/mol | |||||||||||||||||||||||||||||||||
| Heat of vaporization | 400 kJ/mol | |||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.2 J/(mol·K) | |||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||
| Oxidation states | +3 (a strongly basic oxide) | |||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.1 | |||||||||||||||||||||||||||||||||
| Ionization energies |
|
|||||||||||||||||||||||||||||||||
| Covalent radius | 215 pm | |||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) | |||||||||||||||||||||||||||||||||
| Thermal conductivity | 12 W/(m⋅K) | |||||||||||||||||||||||||||||||||
| CAS Number | 7440-34-8 | |||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Friedrich Oskar Giesel (1902, 1903) | |||||||||||||||||||||||||||||||||
| Named by | André-Louis Debierne (1899) | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
